A widely used ADHD medication has been recalled, and patients across the country are being advised to double-check their medicine cabinets.
The announcement has raised concerns about the safety and effectiveness of a drug relied upon by many for daily management of ADHD symptoms.
Questions about how to handle existing prescriptions and what steps to take next are top of mind. Experts emphasize the importance of acting promptly yet cautiously while waiting for official guidance.
The recall details
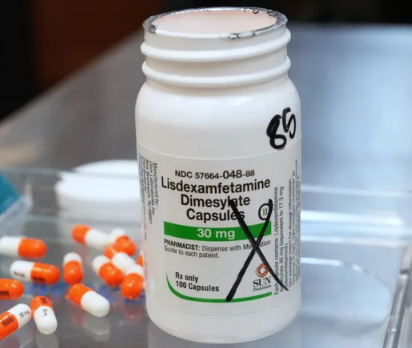
Sun Pharmaceutical Industries, based in New Jersey, voluntarily recalled multiple lots of lisdexamfetamine dimesylate capsules, the generic form of Vyvanse, on October 28, 2025.
The FDA classified the recall as Class II, indicating a low risk of serious health consequences but potential temporary or reversible side effects.
The problem stems from certain batches that “failed dissolution specifications,” meaning the medication may not dissolve properly and could reduce effectiveness.
Patients are urged to check their bottles against the specific lot numbers and expiration dates provided by the manufacturer.
Which products are affected
The recall affects 100-count bottles in strengths ranging from 20 mg to 70 mg. Affected lot numbers include:
- AD42468
- AD42469
- AD42470
- AD48705
- AD48707
- AD48708
- AD48709
- AD50894
- AD48710
- AD50895
- AD48711
- AD50896
- AD48712
- AD50898
with expiration dates from February 28, 2026, to May 31, 2026. No other batches outside these numbers failed testing. Families should carefully compare their prescription bottles with the recall list to determine if their medication is affected.
Also read: Urgent recall affects 2 popular heart medications sold nationwide
Steps to take
It’s important not to stop taking the medication abruptly, as doing so may cause uncomfortable withdrawal symptoms.
Instead, check prescriptions against the recalled lot numbers and contact your pharmacy or prescribing clinician immediately if there is a match.
Health care providers can guide patients on obtaining a replacement bottle or processing a refund. Acting quickly helps ensure uninterrupted treatment.
Also read: FDA announces nationwide recall of over 580,000 bottles of blood pressure medication
Expert guidance and safety Tips
The FDA emphasizes that while the recall poses a low risk of serious health issues, effectiveness may be compromised. Patients should report any adverse reactions or concerns to their health care provider as a precaution.
Maintaining open communication with health care providers ensures treatment remains safe and effective. By following official instructions, patients can minimize stress and keep their ADHD management on track.
Read also:
- FDA issues urgent recall of mislabeled pain medication over life-threatening risks
- Is your cholesterol medication affected by a nationwide recall? Here's what to know
It’s important to take the recall seriously to maintain an uninterrupted treatment schedule. Have you checked the lot numbers on your medication recently? Confirm whether your prescription is affected and act promptly if it is. Share your experience or questions in the comments below.






