A recall alert for Duloxetine: Essential information for those taking this commonly prescribed antidepressant
By
Veronica E.
- Replies 0
In today’s world, the medications we take are often a lifeline to maintaining our well-being and balance. But what happens when those medications, which we trust to support our health, are found to be unsafe?
This is the question many Americans are grappling with following a recent recall of the widely prescribed antidepressant, which has raised concerns across the country.
At The GrayVine, we understand how important it is to stay informed about the medications we rely on, especially when it comes to mental well-being. Let’s take a closer look at this recall and what it could mean for you.
This impurity was found at levels above the recommended interim limit, prompting the recall on November 19.

The U.S. Food and Drug Administration (FDA) has classified this recall as “Class II,” which is one step below the most severe recall classification.
A Class II recall means that exposure to the product may lead to temporary or medically reversible health issues, though the likelihood of serious health consequences is remote.
For many people, Duloxetine is a crucial part of managing their symptoms and improving their quality of life.
If you or a loved one are currently taking Duloxetine, it’s important to check your medication against the recalled lot numbers to ensure your safety.
These compounds can form during the manufacturing or storage process, and long-term exposure to levels above acceptable limits can pose significant health risks.
The FDA and other regulatory agencies have strict guidelines on the allowable levels of these compounds in pharmaceuticals.
This isn’t the first time Duloxetine has been recalled. Back in October, Towa Pharmaceutical Europe also recalled a batch of the medication.
The FDA has explained that nitrosamines can appear in drugs for various reasons, such as during manufacturing, due to chemical structure, or even while the drugs are processed by the body.
1. Check Your Bottles: Compare the lot numbers and expiry dates on your Duloxetine medication with the recalled list.
2. Consult Your Doctor: If your medication is part of the recall, don’t stop taking it suddenly. Reach out to your healthcare provider for advice on alternative treatments or next steps.
3. Stay Informed: Keep an eye out for further updates from the FDA and your pharmacy regarding this recall or additional recalls.

We encourage our community to stay informed and proactive when it comes to their health choices. Have you ever experienced a medication recall or had concerns about the safety of your prescriptions? If you have any concerns or experiences you'd like to share about this recall or medication safety in general, we’d love for you to join the conversation in the comments below!
This is the question many Americans are grappling with following a recent recall of the widely prescribed antidepressant, which has raised concerns across the country.
At The GrayVine, we understand how important it is to stay informed about the medications we rely on, especially when it comes to mental well-being. Let’s take a closer look at this recall and what it could mean for you.
The Recall Rundown: Duloxetine’s Contamination Crisis
Rising Pharmaceuticals, Inc., based in East Brunswick, New Jersey, has voluntarily recalled 233,003 bottles of Duloxetine due to contamination with a potentially cancer-causing chemical known as N-nitroso-duloxetine.This impurity was found at levels above the recommended interim limit, prompting the recall on November 19.

Duloxetine recall: Important safety update for those using this antidepressant. Image Source: Pexels / JESHOOTS.com.
The U.S. Food and Drug Administration (FDA) has classified this recall as “Class II,” which is one step below the most severe recall classification.
A Class II recall means that exposure to the product may lead to temporary or medically reversible health issues, though the likelihood of serious health consequences is remote.
Understanding Duloxetine and Its Uses
Duloxetine is part of a class of drugs called serotonin-norepinephrine reuptake inhibitors (SNRIs). It’s commonly prescribed for treating depression, anxiety, and nerve pain conditions like fibromyalgia.For many people, Duloxetine is a crucial part of managing their symptoms and improving their quality of life.
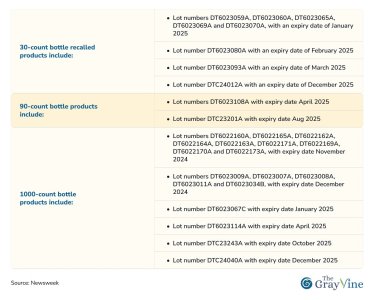
The Specifics: Which Bottles Are Affected?
The recall affects Duloxetine capsules in 30-count, 90-count, and 1000-count bottles, specifically the 60 mg dosage. The lot numbers and expiry dates of the recalled products range from January 2025 to December 2025.If you or a loved one are currently taking Duloxetine, it’s important to check your medication against the recalled lot numbers to ensure your safety.
The N-Nitroso Dilemma: Understanding the Risk
N-nitroso compounds, like the one found in the recalled Duloxetine batches, are classified as potential carcinogens.These compounds can form during the manufacturing or storage process, and long-term exposure to levels above acceptable limits can pose significant health risks.
The FDA and other regulatory agencies have strict guidelines on the allowable levels of these compounds in pharmaceuticals.
This isn’t the first time Duloxetine has been recalled. Back in October, Towa Pharmaceutical Europe also recalled a batch of the medication.
The FDA has explained that nitrosamines can appear in drugs for various reasons, such as during manufacturing, due to chemical structure, or even while the drugs are processed by the body.
Taking Action: What You Should Do Now
If you’re currently taking Duloxetine, here’s what you should do:1. Check Your Bottles: Compare the lot numbers and expiry dates on your Duloxetine medication with the recalled list.
2. Consult Your Doctor: If your medication is part of the recall, don’t stop taking it suddenly. Reach out to your healthcare provider for advice on alternative treatments or next steps.
Here at The GrayVine, we know how unsettling recalls like this can be, especially when they involve medications that are vital to our daily lives. This serves as a powerful reminder to stay vigilant about the medications we take and to trust only those companies that prioritize our health and safety.
Key Takeaways
- A commonly prescribed antidepressant, Duloxetine, has been recalled in the U.S. due to contamination with N-nitroso-duloxetine, a potential carcinogen.
- The recall of 233,003 bottles of Duloxetine by Rising Pharmaceuticals was voluntarily initiated and has been classified as a 'Class II' risk level by the FDA.
- Recalled Duloxetine products include various lot numbers and expiry dates across 30-count, 90-count, and 1000-count bottles of 60 mg capsules.
- Exposure to N-nitroso compounds above interim acceptable limits can pose potential long-term health risks, and regulatory agencies have strict guidelines for acceptable levels in pharmaceuticals.
We encourage our community to stay informed and proactive when it comes to their health choices. Have you ever experienced a medication recall or had concerns about the safety of your prescriptions? If you have any concerns or experiences you'd like to share about this recall or medication safety in general, we’d love for you to join the conversation in the comments below!