FDA warning: Hidden risks in popular asthma medication
By
Aubrey Razon
- Replies 0
Disclaimer: This article discusses topics related to mental health, including suicide. Reader discretion is advised, and if you are struggling with these issues, please seek support from a healthcare professional.
Medications we trust could have surprising effects we never expect. A new warning from the U.S. Food and Drugs Administration (FDA) has uncovered a hidden risk in a widely used asthma drug—could it affect you?
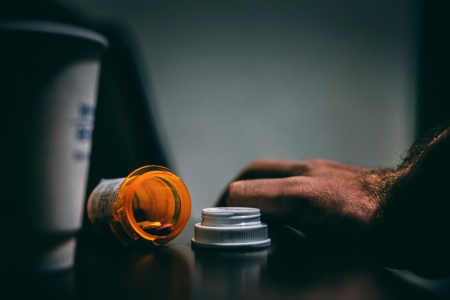
Singulair, known generically as montelukast, has been a go-to prescription for asthma and allergy sufferers since its launch in 1998.
It's designed to ease breathing by reducing inflammation in the airways. However, a shadow has been cast over this seemingly helpful drug.
The FDA has presented preliminary research suggesting that Singulair may bind to brain receptors linked to mood, impulse control, cognition, sleep, and more.
This binding is “definitely doing something that's concerning,” according to Julia Marschallinger, a scientist at Austria's Institute of Molecular Regenerative Medicine.
While the exact implications of this interaction are not fully understood, the FDA's findings are enough to warrant a closer look at the medication many have trusted for over two decades.
The side effects associated with Singulair are not to be taken lightly.
They range from anxiety, nervousness, and confusion to more severe issues like hallucinations, irritability, hostility, and even thoughts of suicide or self-harm.
Depression and sleep disturbances, including vivid dreams or nightmares, have also been reported.
Despite previous warnings about the risk of suicidal thoughts or actions, the message hadn't reached everyone who needed to hear it.
For those in our community who are taking Singulair or its generic versions, it's imperative to be aware of these potential side effects.
If you or someone you know is experiencing any of these symptoms, it's crucial to contact your healthcare provider immediately.
The FDA's concerns about Singulair are not new.
In 2020, the agency added a Boxed Warning to the drug, indicating serious mental health side effects and suggesting restricted use for hay fever.
In October, the FDA took further action by listing the drug as having serious risks or new safety information.
This move signals the agency's ongoing evaluation of the need for regulatory action.
The recent revelations about Singulair serve as a reminder to stay proactive about our health. Here's what you can do:
1. Review Your Medications: Take stock of all the medications you're currently taking, including Singulair. Be aware of their potential side effects.
2. Consult Your Doctor: If you're concerned about Singulair, talk to your healthcare provider about alternative asthma treatments that may have a safer side effect profile.
3. Stay Informed: Keep up with the latest FDA updates and research findings related to your medications.
4. Share Your Experience: If you've had an adverse reaction to Singulair, report it to the FDA's MedWatch program to contribute to the body of knowledge about the drug's safety.
5. Support Each Other: If you know someone in our community who's struggling with mental health issues potentially linked to medication, offer your support and encourage them to seek professional help.
In a previous story, glass particles were found in vitamins which sparked a massive recall. Read more about this story here.
 Have you or a loved one experienced side effects while taking Singulair? How do you stay informed about the medications you take? Share your stories and tips in the comments below.
Have you or a loved one experienced side effects while taking Singulair? How do you stay informed about the medications you take? Share your stories and tips in the comments below.
Medications we trust could have surprising effects we never expect. A new warning from the U.S. Food and Drugs Administration (FDA) has uncovered a hidden risk in a widely used asthma drug—could it affect you?
Singulair, known generically as montelukast, has been a go-to prescription for asthma and allergy sufferers since its launch in 1998.
It's designed to ease breathing by reducing inflammation in the airways. However, a shadow has been cast over this seemingly helpful drug.
The FDA has presented preliminary research suggesting that Singulair may bind to brain receptors linked to mood, impulse control, cognition, sleep, and more.
This binding is “definitely doing something that's concerning,” according to Julia Marschallinger, a scientist at Austria's Institute of Molecular Regenerative Medicine.
While the exact implications of this interaction are not fully understood, the FDA's findings are enough to warrant a closer look at the medication many have trusted for over two decades.
The side effects associated with Singulair are not to be taken lightly.
They range from anxiety, nervousness, and confusion to more severe issues like hallucinations, irritability, hostility, and even thoughts of suicide or self-harm.
Depression and sleep disturbances, including vivid dreams or nightmares, have also been reported.
Despite previous warnings about the risk of suicidal thoughts or actions, the message hadn't reached everyone who needed to hear it.
For those in our community who are taking Singulair or its generic versions, it's imperative to be aware of these potential side effects.
If you or someone you know is experiencing any of these symptoms, it's crucial to contact your healthcare provider immediately.
The FDA's concerns about Singulair are not new.
In 2020, the agency added a Boxed Warning to the drug, indicating serious mental health side effects and suggesting restricted use for hay fever.
In October, the FDA took further action by listing the drug as having serious risks or new safety information.
This move signals the agency's ongoing evaluation of the need for regulatory action.
The recent revelations about Singulair serve as a reminder to stay proactive about our health. Here's what you can do:
1. Review Your Medications: Take stock of all the medications you're currently taking, including Singulair. Be aware of their potential side effects.
2. Consult Your Doctor: If you're concerned about Singulair, talk to your healthcare provider about alternative asthma treatments that may have a safer side effect profile.
3. Stay Informed: Keep up with the latest FDA updates and research findings related to your medications.
4. Share Your Experience: If you've had an adverse reaction to Singulair, report it to the FDA's MedWatch program to contribute to the body of knowledge about the drug's safety.
5. Support Each Other: If you know someone in our community who's struggling with mental health issues potentially linked to medication, offer your support and encourage them to seek professional help.
In a previous story, glass particles were found in vitamins which sparked a massive recall. Read more about this story here.
Key Takeaways
- The popular asthma medication Singulair, also known generically as montelukast, may be linked to mental health side effects and suicides according to a recent FDA study.
- Laboratory tests have shown significant binding of Singulair to multiple brain receptors that govern mood, impulse control, cognition and sleep, although further research is required to determine if this leads to harmful side effects.
- Despite concerns and earlier warnings, the FDA will not be updating the drug's label with the new data presented.
- Singulair has been under scrutiny after reports of neuropsychiatric episodes and dozens of suicides in patients taking the medication, resulting in the FDA requiring a Boxed Warning on the drug alerting to its serious mental health side effects.