Over 14,000 cold and flu products recalled—are you at risk?
- Replies 1
In homes across the country, the medicine cabinet is a trusted first stop for cold and flu relief.
But now, more than 14,000 boxes of a popular over-the-counter remedy are under recall—posing a potential health risk to families, especially those with young children
If you’ve stocked up on cold and flu medicine this season, it’s time to take a closer look.
The medicine in question is Safetussin Max Strength Multi-Symptom Cough, Cold and Flu caplets.
Sold in 24-count blister packs, this product was pulled from store shelves due to a packaging defect: it lacks the federally required child-resistant features.
According to the Consumer Product Safety Commission (CPSC), the medicine’s packaging fails to meet standards outlined in the Poison Prevention Packaging Act, which helps prevent accidental ingestion by young children.
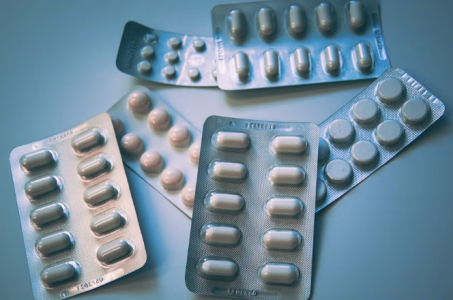
Without proper safety mechanisms, kids can push the tablets through the foil and swallow them—putting them at risk of serious harm.
This cold and flu product was sold in regional grocery stores and pharmacies nationwide, including HEB and Harris-Teeter, between July 2024 and March 2025. It retailed for approximately $11.
If you’ve purchased medication during this timeframe, check the box carefully. Look for blue, red, and orange packaging with dosage and ingredient information on the back.
Medicines like Safetussin often contain acetaminophen, a powerful fever reducer that can be dangerous—even deadly—if taken in high amounts.
The child-resistant packaging rule isn’t just a formality—it’s a safeguard that’s helped reduce unintentional poisonings since the 1970s.
If you find this product in your home, stop using it immediately. You can either dispose of it safely or return it to the store for a full refund.
The manufacturer, Kramer Laboratories, is available for questions at 800-824-4894 (Monday–Friday, 8 a.m.–5 p.m. EDT) or via email at [email protected].
If a child has accidentally taken the medicine, call Poison Control immediately at 1-800-222-1222, or dial 911 in an emergency.
Read more:
 Have you found this medicine in your cabinet? Do you have tips for safely storing medications in your home? Or questions about how to handle recalled products? Share your stories in the comments—we’d love to hear how you’re protecting your family.
Have you found this medicine in your cabinet? Do you have tips for safely storing medications in your home? Or questions about how to handle recalled products? Share your stories in the comments—we’d love to hear how you’re protecting your family.
But now, more than 14,000 boxes of a popular over-the-counter remedy are under recall—posing a potential health risk to families, especially those with young children
If you’ve stocked up on cold and flu medicine this season, it’s time to take a closer look.
The medicine in question is Safetussin Max Strength Multi-Symptom Cough, Cold and Flu caplets.
Sold in 24-count blister packs, this product was pulled from store shelves due to a packaging defect: it lacks the federally required child-resistant features.
According to the Consumer Product Safety Commission (CPSC), the medicine’s packaging fails to meet standards outlined in the Poison Prevention Packaging Act, which helps prevent accidental ingestion by young children.

Over 14,000 boxes of Safetussin Max Strength Multi-Symptom Cough, Cold and Flu medicine have been recalled. Image source: Christine Sandu / Unsplash
Without proper safety mechanisms, kids can push the tablets through the foil and swallow them—putting them at risk of serious harm.
This cold and flu product was sold in regional grocery stores and pharmacies nationwide, including HEB and Harris-Teeter, between July 2024 and March 2025. It retailed for approximately $11.
If you’ve purchased medication during this timeframe, check the box carefully. Look for blue, red, and orange packaging with dosage and ingredient information on the back.
Medicines like Safetussin often contain acetaminophen, a powerful fever reducer that can be dangerous—even deadly—if taken in high amounts.
The child-resistant packaging rule isn’t just a formality—it’s a safeguard that’s helped reduce unintentional poisonings since the 1970s.
If you find this product in your home, stop using it immediately. You can either dispose of it safely or return it to the store for a full refund.
The manufacturer, Kramer Laboratories, is available for questions at 800-824-4894 (Monday–Friday, 8 a.m.–5 p.m. EDT) or via email at [email protected].
If a child has accidentally taken the medicine, call Poison Control immediately at 1-800-222-1222, or dial 911 in an emergency.
Read more:
- Recall alert: Something’s not right with this popular dairy product
- This fizzy favorite just got a recall of more than 10,000 cans—here’s why
Key Takeaways
- Over 14,000 boxes of Safetussin Max Strength Multi-Symptom Cough, Cold and Flu medicine have been recalled.
- The recalled medicine lacks the required child-resistant packaging, which could pose a risk of poisoning to young children.
- The medicine was distributed in several regional grocery stores and pharmacies across the nation and was sold from July 2024 through March 2025.
- Consumers who have purchased the product are advised to dispose of it or return it for a refund, and contact Poison Control or seek emergency assistance if a child has accessed the medication.






